Sinopharm Vaccine Wikipedia : Sinopharm Bibp Covid 19 Vaccine Wikipedia
The Sinopharm jab is an inactivated vaccine containing a killed coronavirus that cannot replicate. The interim recommendations and the background document are also available online.

China S Covid Vaccine From Sinopharm Is 86 Effective Uae Says
This article provides a summary of the interim recommendations.

Sinopharm vaccine wikipedia. CoronaVac also known as the Sinovac COVID-19 vaccine is an inactivated virus COVID-19 vaccine developed by the Chinese company Sinovac Biotech. A COVID19 vaccine is a vaccine intended to provide acquired immunity against severe acute respiratory syndrome coronavirus 2 SARSCoV2 the virus that causes coronavirus disease 2019 Prior to the COVID19 pandemic an established body of knowledge existed about the structure and function of coronaviruses causing diseases like severe acute respiratory syndrome SARS and Middle. This is a different approach to the mRNA-based vaccines of Pfizer and Moderna and the viral.
Clinical trials run by the state-owned. The WHO Strategic Advisory Group of Experts SAGE on Immunization has issued Interim recommendations for the use of the inactivated COVID-19 vaccine BIBP developed by SinopharmChina National Pharmaceutical Group. By May Sinopharm had supplied 200 million doses.
Sinopharm expects to produce one billion doses of BBIBP-CorV in 2021. Other licensed vaccines that use this type of technology. It completed Phase III trials in Argentina Bahrain Egypt Morocco Pakistan Peru and the United Arab Emirates UAE with over 60000 participants.
Sinopharm vero cell vaccine efficacy. Lina said on the Chinese social media platform Weibo that the COVID-19 vaccine developed by Sinopharm which is a Chinese state-run company is extremely unsafe for use according to Daily Mail. Number of doses required.
This vaccine does not have a name. So far Vero is the only Chinese vaccine for which the manufacturer has published official data. On 7 May 2021 the World Health Organization approved the vaccine for use in COVAX.
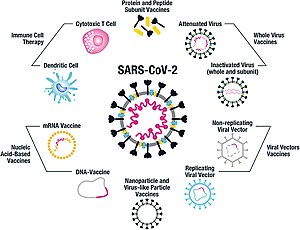
Its product name is SARS-CoV-2 Vaccine Vero Cell not to be confused with the similar product name of CoronaVac. The whole virus vaccine uses a weakened or deactivated form of the pathogen that causes COVID-19 to trigger protective immunity to it. This chemical binds to the viruss genetic material and stops it from.
Bacillus Calmette-Guerin BRACE trial Edit. The Sinopharm vaccine contains SARS-CoV-2 that has undergone treatment with a chemical called beta-propiolactone. On late July and early August 2021 Ho Chi Minh City received 2 million of the 5 million doses of Sinopharms Vero Cell vaccine purchased by Sapharco.
Hepatitis A polio rabies all inactivated type What to know. By May Sinopharm had supplied 200 million doses. Sinopharm expects to produce one billion doses of BBIBP-CorV in 2021.
A BBIBP-CorV vagy a Sinopharm Covid19-vakcina a köznyelvben kínai vakcina a Pekingi Biológiai Termékek Intézete Beijing Institute of Biological Products által kifejlesztett és a kínai Sinopharm által gyártott elsőgenerációs teljes elölt SARS-CoV-2 vírust tartalmazó kétdózisú Covid19-vakcina mely a Covid19 megbetegedés ellen nyújt védelmet. On 7 May 2021 the World Health Organization approved the vaccine for use in COVAX. The company Sinopharm belongs to the government of China.
The OxfordAstraZeneca COVID-19 vaccine sold under the brand names Vaxzevria and Covishield is a viral vector vaccine produced by the British University of Oxford British-Swedish company AstraZeneca and the Coalition for Epidemic Preparedness Innovations. Sinopharm vaccine will be administered to Chinese nationals in Vietnam those wishing to go to study or work in China and those living in border areas with China. The other inactivated virus vaccine developed by Sinopharm is WIBP-CorV.
How the Sinopharm Vaccine Works. Denmark and Norway suspended the use of the OxfordAstraZeneca vaccine due to a small number of reports of a rare blood clot disorder. It was Phase III clinical trialled in Brazil Chile Indonesia the Philippines and Turkey and relies on traditional technology similar to BBIBP-CorV and Covaxin other inactivated-virus COVID-19 vaccines.
Sinopharm BBIBP-CorV also known as the Sinopharm COVID-19 vaccine is one of two inactivated virus COVID-19 vaccines developed by Sinopharm. It is an inactivated virus vaccine. CoronaVac does not need to be frozen and.
The other inactivated virus vaccine developed by Sinopharm is WIBP-CorV. EXPERIMENTAL COVID-19 VACCINES BIO-WEAPONS. In early 2020 the Beijing Institute of Biological Products created an inactivated coronavirus vaccine called BBIBP-CorV.
The document has moved here. This vaccine started phase III trials in the middle of July 2020. Here is what you need to know.
On December 29 2020 Sinopharm reported 79 efficacy in an interim evaluation.

Why Does Africa Not Have Its Own Covid 19 Vaccine Quartz Africa

Who Approval Of Chinese Vaccine Will Largely Accelerate Covax Supply Analysts Global Times

The Cold War Diplomacy Behind Covid 19 Vaccines

Sinopharm Bibp Covid 19 Vaccine Wikipedia
Chinese State Backed Firm Expects Coronavirus Vaccine Approval For Public Use Within Months Reuters
Pakistan China To Gift Half Million Doses Of Sinopharm Vaccine Reuters

Sinopharm Vero Cell Inactivated Covid 19 Vaccine

Uae Says Sinopharm Vaccine Has 86pc Efficacy Against Covid 19 World Dawn Com
/cloudfront-us-east-2.images.arcpublishing.com/reuters/K7IMX7V5NZOOVGSOPKVPNUA6MY.jpg)
Sinopharm S Two Covid 19 Shots Effective Study Says Reuters

Sinopharm Bibp Covid 19 Vaccine Wikipedia
Zimbabwe To Get 800 000 Doses Of Chinese Sinopharm Vaccine

Pfizer Biontech Covid 19 Vaccine Wikipedia

Oxford Astrazeneca Covid 19 Vaccine Wikipedia
Chinese Vaccine Candidate Effective Against All Known Coronavirus Strains Sinopharm Global Times